JOINTREP™
CARTILAGE REGENERATION TECHNOLOGY
JointRep™ is being tested in a controlled clinical study in Europe, to compare a JointRep™ + microfracture operation to an operation with standard microfracture alone, on 69 patients of ages 18-75 with grade III-IV cartilage lesions unrestricted in size.
Controlled post-market clinical study for use of JointRep™ in conjunction with microfracture in grade III-IV knee cartilage lesions showed exceptional results in terms of pain reduction and mobility improvement for patients, and evidence of hyaline cartilage regrowth.
Analysis of WOMAC mean Scores
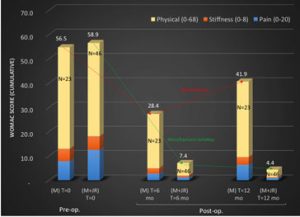
The mean initial WOMAC score in the test group was reduced by 88% at 6 months and 93% at 12 months (Please refer to the diagram above)
The in-vitro study
The in-vitro study found general characteristics of hyaline cartilage in test cells: rounded shape cells trapped inside lacunae and type II collagen. Control cells produced a fibrous cartilage-like tissue
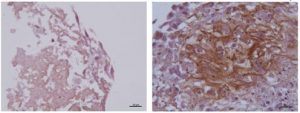
Figure 1: Human collagen type II immunostaining negative in control samples (left) and positive in the extracellular matrix of induced cells (right) seeded in JointRep™ solution
The Biopsy
The biopsy also revealed hyaline-like cartilage in the regenerated area
The entire femoral bone-cartilage site, before histological processing, was macroscopically examined: a general overview evidences the glass-like aspect of articular hyaline cartilage with restored smooth and white cartilage covering surface of the distal femur, as shown in Figure 2.
The matrix is abundant and well evidenced by histological staining. The deepest zone contains calcified cartilage and the subchondral bone evidenced by the intense red staining.
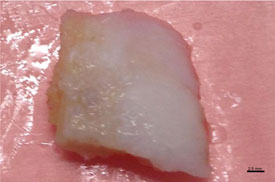
Figure 2. Macroscopic view of human femoral condyle